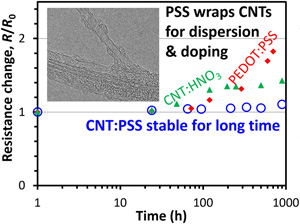
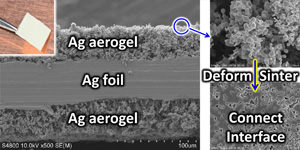
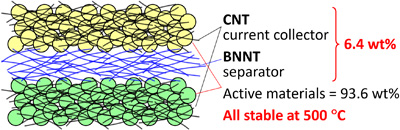
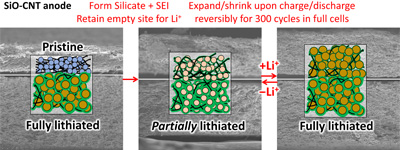
On the other hand, as their price (higher than gold) shows, their fabrication process is still under development and their practical applications are very much limited. Chemistry & engineering should lead the innovations for their production and manufacturing. We have developed rapid growth process of millimeter-long single-wall CNTs and are trying to realize their practical production. Please click here for details.
We are developing mass-production processes of CNTs by utilizing three-dimensional space of reactors, and direct fabrication of various devices by growing CNTs on device substrates. We have also started the synthesis of boron nitride nanotubes (BNNTs) having similar structure as CNTs and insulative property.
- Pengfei CHEN (D3): Activation of alkane for CVD synthesis of CNTs.
- Shun TANAKA (M2): Development of a safe and low-damage dry purification method for CNTs.
- Yuki NAOTSUKA (M2): Stable and continuous synthesis of carbon nanotubes by floating catalyst CVD with enhanced decomposition of catalyst sources
- Koyo ANDO (M1): Synthesis of CNTs by floating catalyst CVD method and development of transparent thin films.
- Daiki KITAJIMA (M1): Coating of carbon nanotube with boron nitride for lithium-ion battery application.
- Koki AKIYAMA (B4): Morphology control of vertically aligned CNT films and application to electron emitters for small X-ray tubes.
- Yoshiyuki MATSUKAWA (B4): Fluidized bed synthesis of long, high-purity CNTs and co-production of low-carbon fuels.

Rapid SWCNT growth [58]. Larger Movie.

Continuous production by fluidized bed [60].
Watch detailed version here.